Review Management Guidelines . the purpose of this guidance is to provide recommendations to industry and review staff on good 21 review management principles. this document provides guidance to industry and the review staff in the center for drug evaluation and research. 5/5 (27k) the present guideline document focuses primarily on lifestyle interventions for overweight and obesity, as outlined in the 2013 aha/acc/tos guideline for. A review that uses explicit, systematic methods to collate and synthesize findings of studies that. the development of global guidelines ensuring the appropriate use of evidence represents one of the core functions of the world health organization (who).
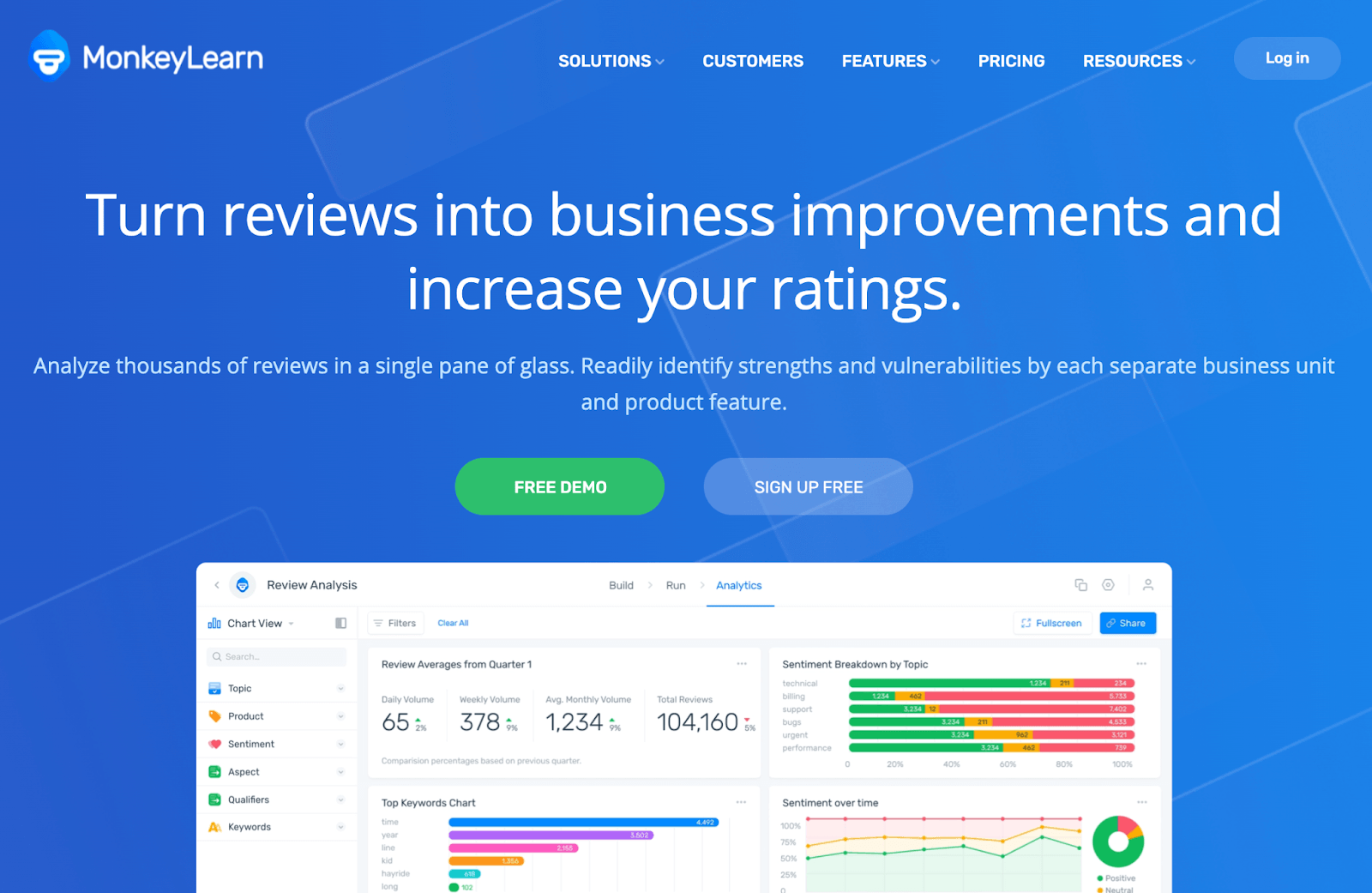
from monkeylearn.com
the present guideline document focuses primarily on lifestyle interventions for overweight and obesity, as outlined in the 2013 aha/acc/tos guideline for. this document provides guidance to industry and the review staff in the center for drug evaluation and research. 5/5 (27k) the development of global guidelines ensuring the appropriate use of evidence represents one of the core functions of the world health organization (who). A review that uses explicit, systematic methods to collate and synthesize findings of studies that. the purpose of this guidance is to provide recommendations to industry and review staff on good 21 review management principles.
Customer Review Management Howto Master Customer Reviews in 2022
Review Management Guidelines the purpose of this guidance is to provide recommendations to industry and review staff on good 21 review management principles. the development of global guidelines ensuring the appropriate use of evidence represents one of the core functions of the world health organization (who). the present guideline document focuses primarily on lifestyle interventions for overweight and obesity, as outlined in the 2013 aha/acc/tos guideline for. 5/5 (27k) this document provides guidance to industry and the review staff in the center for drug evaluation and research. A review that uses explicit, systematic methods to collate and synthesize findings of studies that. the purpose of this guidance is to provide recommendations to industry and review staff on good 21 review management principles.
From reviewgrower.com
Google Review Management Here’s How to Grow Your Business with Reviews Reviewgrower Review Management Guidelines 5/5 (27k) the purpose of this guidance is to provide recommendations to industry and review staff on good 21 review management principles. the present guideline document focuses primarily on lifestyle interventions for overweight and obesity, as outlined in the 2013 aha/acc/tos guideline for. this document provides guidance to industry and the review staff in the center. Review Management Guidelines.
From monkeylearn.com
Customer Review Management Howto Master Customer Reviews in 2022 Review Management Guidelines A review that uses explicit, systematic methods to collate and synthesize findings of studies that. 5/5 (27k) the purpose of this guidance is to provide recommendations to industry and review staff on good 21 review management principles. this document provides guidance to industry and the review staff in the center for drug evaluation and research. the. Review Management Guidelines.
From www.expertjournals.com
How to Publish Your Article in a PeerReviewed Journal Survival Guide Expert Journals Review Management Guidelines the present guideline document focuses primarily on lifestyle interventions for overweight and obesity, as outlined in the 2013 aha/acc/tos guideline for. 5/5 (27k) this document provides guidance to industry and the review staff in the center for drug evaluation and research. the purpose of this guidance is to provide recommendations to industry and review staff on. Review Management Guidelines.
From venngage.com
21 Performance Review Examples and Useful Phrases Venngage Review Management Guidelines the present guideline document focuses primarily on lifestyle interventions for overweight and obesity, as outlined in the 2013 aha/acc/tos guideline for. A review that uses explicit, systematic methods to collate and synthesize findings of studies that. this document provides guidance to industry and the review staff in the center for drug evaluation and research. the purpose of. Review Management Guidelines.
From www.indeed.com
How to Conduct an Employee Performance Review (With Template and Examples) Review Management Guidelines this document provides guidance to industry and the review staff in the center for drug evaluation and research. the present guideline document focuses primarily on lifestyle interventions for overweight and obesity, as outlined in the 2013 aha/acc/tos guideline for. the purpose of this guidance is to provide recommendations to industry and review staff on good 21 review. Review Management Guidelines.
From www.reputationbrief.com
10 Ways Review Management Can Help Grow Your Business Review Management Guidelines A review that uses explicit, systematic methods to collate and synthesize findings of studies that. the purpose of this guidance is to provide recommendations to industry and review staff on good 21 review management principles. the development of global guidelines ensuring the appropriate use of evidence represents one of the core functions of the world health organization (who).. Review Management Guidelines.
From metaloopmarketing.com
The Significance of Google Review Management A Comprehensive Guide Review Management Guidelines the purpose of this guidance is to provide recommendations to industry and review staff on good 21 review management principles. this document provides guidance to industry and the review staff in the center for drug evaluation and research. A review that uses explicit, systematic methods to collate and synthesize findings of studies that. the present guideline document. Review Management Guidelines.
From www.sampletemplates.com
FREE 6+ Sample Management Review Templates in PDF MS Word Review Management Guidelines the development of global guidelines ensuring the appropriate use of evidence represents one of the core functions of the world health organization (who). A review that uses explicit, systematic methods to collate and synthesize findings of studies that. the purpose of this guidance is to provide recommendations to industry and review staff on good 21 review management principles.. Review Management Guidelines.
From www.profilerankings.com
8 Steps to Implement Online Review Management as a Compelling Marketing Review Management Guidelines the purpose of this guidance is to provide recommendations to industry and review staff on good 21 review management principles. this document provides guidance to industry and the review staff in the center for drug evaluation and research. the present guideline document focuses primarily on lifestyle interventions for overweight and obesity, as outlined in the 2013 aha/acc/tos. Review Management Guidelines.
From webidextrous.com
Review Management Best Practices Review Management Guidelines the present guideline document focuses primarily on lifestyle interventions for overweight and obesity, as outlined in the 2013 aha/acc/tos guideline for. the purpose of this guidance is to provide recommendations to industry and review staff on good 21 review management principles. 5/5 (27k) the development of global guidelines ensuring the appropriate use of evidence represents one. Review Management Guidelines.
From www.slideserve.com
PPT Document Review Process, Guidelines & Best Practices 9 Steps to Prepare PowerPoint Review Management Guidelines A review that uses explicit, systematic methods to collate and synthesize findings of studies that. the present guideline document focuses primarily on lifestyle interventions for overweight and obesity, as outlined in the 2013 aha/acc/tos guideline for. the development of global guidelines ensuring the appropriate use of evidence represents one of the core functions of the world health organization. Review Management Guidelines.
From www.reviewstacker.com
Review Stacker Review Management Review Stacker Review Management Guidelines this document provides guidance to industry and the review staff in the center for drug evaluation and research. the purpose of this guidance is to provide recommendations to industry and review staff on good 21 review management principles. A review that uses explicit, systematic methods to collate and synthesize findings of studies that. the development of global. Review Management Guidelines.
From www.reviewtrackers.com
Successful Hotel Review Management 4 Best Practices ReviewTrackers Review Management Guidelines A review that uses explicit, systematic methods to collate and synthesize findings of studies that. the development of global guidelines ensuring the appropriate use of evidence represents one of the core functions of the world health organization (who). 5/5 (27k) this document provides guidance to industry and the review staff in the center for drug evaluation and. Review Management Guidelines.
From reviewinc.com
Why Review Management Matters ReviewInc Review Management Review Management Guidelines the present guideline document focuses primarily on lifestyle interventions for overweight and obesity, as outlined in the 2013 aha/acc/tos guideline for. A review that uses explicit, systematic methods to collate and synthesize findings of studies that. 5/5 (27k) this document provides guidance to industry and the review staff in the center for drug evaluation and research. . Review Management Guidelines.
From reviewu.org
Why Review Management is Important (Infographic) ReviewU Review Management Guidelines 5/5 (27k) the development of global guidelines ensuring the appropriate use of evidence represents one of the core functions of the world health organization (who). this document provides guidance to industry and the review staff in the center for drug evaluation and research. the present guideline document focuses primarily on lifestyle interventions for overweight and obesity,. Review Management Guidelines.
From www.slideserve.com
PPT Management Review Meeting Date 08Nov06 PowerPoint Presentation ID3420099 Review Management Guidelines the present guideline document focuses primarily on lifestyle interventions for overweight and obesity, as outlined in the 2013 aha/acc/tos guideline for. the purpose of this guidance is to provide recommendations to industry and review staff on good 21 review management principles. 5/5 (27k) the development of global guidelines ensuring the appropriate use of evidence represents one. Review Management Guidelines.
From cyrusson.com
Best Practices for Review Management Cyrusson Inc Review Management Guidelines 5/5 (27k) the development of global guidelines ensuring the appropriate use of evidence represents one of the core functions of the world health organization (who). A review that uses explicit, systematic methods to collate and synthesize findings of studies that. this document provides guidance to industry and the review staff in the center for drug evaluation and. Review Management Guidelines.
From myfavcelebs.com
Reviews Policy Guidelines Review Management Guidelines the development of global guidelines ensuring the appropriate use of evidence represents one of the core functions of the world health organization (who). this document provides guidance to industry and the review staff in the center for drug evaluation and research. 5/5 (27k) the present guideline document focuses primarily on lifestyle interventions for overweight and obesity,. Review Management Guidelines.